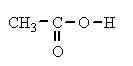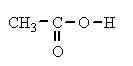acetic acid , CH
3CO
2H, colorless
liquid that has a characteristic pungent odor, boils at 118°C, and is miscible
with water in all proportions; it is a weak organic carboxylic acid (see
carboxyl group). Glacial acetic acid is
concentrated, 99.5% pure acetic acid; it solidifies at about 17°C to a
crystalline mass resembling ice. Acetic acid is the major acid in
vinegar; as such, it is widely used as a food
preservative and condiment. For industrial use concentrated acetic acid is
prepared from the oxidation of
acetaldehyde. Acetic acid is also a product in
the destructive distillation of wood. It reacts with other chemicals to form
numerous compounds of commercial importance. These include cellulose acetate,
used in making acetate rayon, nonflammable motion-picture film, lacquers, and
plastics; various inorganic salts, e.g., lead, potassium, and copper acetates;
and amyl, butyl, ethyl, methyl, and propyl
acetates, which are used as solvents,
chiefly in certain quick-drying lacquers and cements. Amyl acetate is sometimes
called banana oil because it has a characteristic banana odor.
The Columbia Electronic Encyclopedia Copyright c
1994, 2000, Columbia University Press. Licensed from Columbia University Press.
All rights reserved.